.
This law was first formulated by German chemist Walther Nernst in 1906. According to this law,
“The entropy of all perfectly crystalline solids is zero at the absolute zero temperature. Since entropy is a measure of disorder, it can be interpretated that at absolute zero, a perfectly crystalline solid has a perfect order of its constituent particles.”
This law was first formulated by German chemist Walther Nernst in 1906. According to this law,
“The entropy of all perfectly crystalline solids is zero at the absolute zero temperature. Since entropy is a measure of disorder, it can be interpretated that at absolute zero, a perfectly crystalline solid has a perfect order of its constituent particles.”
The most important application of the third law of thermodynamics is that it helps in the calculation of absolute entropies of the substance at any temperature T.
Where 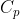 is the heat capacity of the substance at constant pressure and is supposed to remain constant in the range of 0 to T.
is the heat capacity of the substance at constant pressure and is supposed to remain constant in the range of 0 to T.
Limitation of Law:
Limitation of Law:
- Glassy solids even at
has entropy greater than zero.
- Solids having mixtures of isotopes do not have zero entropy at
. For example, entropy of solid chlorine is not zero at
.
- Crystals of
, etc. do not have perfect order even at
thus their entropy is not equal to zero.
No comments:
Post a Comment