According to Arrhenius concept all substances which give 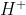 ions when dissolved in water are called acids while those which ionize in water to furnish
ions when dissolved in water are called acids while those which ionize in water to furnish 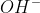 ions are called bases.
ions are called bases.
Some acids and bases ionize almost completely in solutions and are called strong acids and bases. Others are dissociated to a limited extent in solutions and are termed weak acids and bases. HCl, 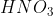 ,
, 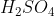 ,
, 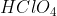 , etc., are examples of strong acids and NaOH, KOH,
, etc., are examples of strong acids and NaOH, KOH, 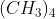 NOH are strong bases. Every hydrogen compound cannot be regarded as an acid, e.g.,
NOH are strong bases. Every hydrogen compound cannot be regarded as an acid, e.g., 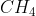 is not an acid. Similarly,
is not an acid. Similarly, 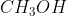 ,
, 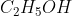 , etc., have OH groups but they are not bases.
, etc., have OH groups but they are not bases.
(i) Utility of Arrhenius concept :
The Arrhenius concept of acids and bases was able to explain a number of phenomenon like neutralization, salt hydrolysis, strength of acids and bases etc.
(ii) Limitations of Arrhenius concept:
- For the acidic or basic properties, the presence of water is absolutely necessary. Dry HCl shall not act as an acid. HCl is regarded as an acid only when dissolved in water and not in any other solvent.
- The concept does not explain acidic and basic character of substances in non-aqueous solvents.
- The neutralization process is limited to those reactions which can occur in aqueous solutions only, although reactions involving salt formation do occur in absence of solvent.
- It cannot explain the acidic character of certain salts such as AlCl3 in aqueous solution.
No comments:
Post a Comment