.
It is the amount of heat evolved (i.e., change in enthalpy) when one equivalent of an acid is neutralized by one equivalent of a base in fairly dilute solution, e.g., Neutralization reactions are always exothermic reaction and the value of H is (–ve).
It is the amount of heat evolved (i.e., change in enthalpy) when one equivalent of an acid is neutralized by one equivalent of a base in fairly dilute solution, e.g., Neutralization reactions are always exothermic reaction and the value of H is (–ve).
HCl(aq.) + NaOH (aq.) –> NaCl(aq.) + H2O
The heat of neutralization of a strong acid against a strong base is always constant .
In case of neutralization of a weak acid or a weak base against a strong base or acid respectively, since a part of the evolved heat is used up in ionizing the weak acid or base, it is always less than 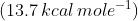
 .
.
For example,
For example,
10.8 kcal of heat is absorbed for ionization of HCN it is heat of dissociation or ionization.
.
No comments:
Post a Comment