Rutherford and Soddy, in 1903, postulated that radioactivity is a nuclear phenomenon and all the radioactive changes are taking place in the nucleus of the atom. They presented an interpretation of the radioactive processes and the origin of radiations in the form of a theory known as theory of radioactive decay. The main points of this theory are,
- The atomic nuclei of the radioactive elements are unstable and liable to disintegrate any moment.
- The disintegration is spontaneous, i.e., constantly breaking. The rate of breaking is not affected by external factors like temperature, pressure, chemical combination etc.
- During disintegration, atoms of new elements called daughter elements having different physical and chemical properties than the parent elements come into existence.
- During disintegration, either alpha or beta particles are emitted from the nucleus.
The disintegration process may proceed in one of the following two ways,
(1) Alpha particle emission :
When an α -particle 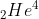 is emitted from the nucleus of an atom of the parent element, the nucleus of the new element, called daughter element possesses atomic mass or atomic mass number less by four units and nuclear charge or atomic number less by 2 units because a-particle has mass of 4 units and nuclear charge of two units.
is emitted from the nucleus of an atom of the parent element, the nucleus of the new element, called daughter element possesses atomic mass or atomic mass number less by four units and nuclear charge or atomic number less by 2 units because a-particle has mass of 4 units and nuclear charge of two units.
(ii) Beta particle emission :
β-particle is merely an electron which has negligible mass. Whenever a beta particle is emitted from the nucleus of a radioactive atom, the nucleus of the new element formed possesses the same atomic mass but nuclear charge or atomic number is increased by 1 unit than the parent element. Beta particle emission is due to the result of decay of neutron into proton and electron.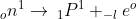
The electron produced escapes as a beta-particle-leaving proton in the nucleus.
(iii) Gamma ray emission :
γ-rays are emitted due to secondary effects. The excess of energy is released in the form of γ-rays. Thus γ-rays arise from energy re-arrangements in the nucleus. As γ-rays are short wavelength electromagnetic radiations with no charge and no mass, their emission from a radioactive element does not produce new element.
Special case :If in a radioactive transformation 1 alpha and 2 beta-particles are emitted, the resulting nucleus possesses the same atomic number but atomic mass is less by 4 units. A radioactive transformation of this type always produces an isotope of the parent element.
A and D are isotopes.
.
No comments:
Post a Comment