Sublimation is a process in which a solid on heating changes directly into gaseous state below its melting point.
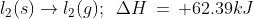
Heat of sublimation of a substance is the amount of heat absorbed in the conversion of 1 mole of a solid directly into vapor phase at a given temperature below its melting point.
Most solids that sublime are molecular in nature e.g. iodine and naphthalene etc.
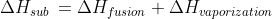
.
No comments:
Post a Comment