Statement:
When the same quantity of electricity is passed through different electrolytes, the masses of the elements liberated or deposited are in proportion to the chemical equivalents of these elements. Faraday's laws are very useful for the determination of electrochemical equivalents of different substances.
Chemical Equivalent:
Explanation:
According to Faraday, if 96,500 Coulombs (or 1 Faraday) is passed through these electrolytes,
we get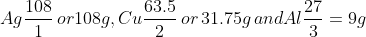 which are the equivalent masses of
which are the equivalent masses of
Ag, Cu and Al respectively.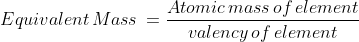
.
The chemical equivalent of an element is numerically equal to its relative atomic mass in grams divided by its the valency of the ion.
Faraday's second law of electrolysis can also be stated as under:
"The mass of different substances liberated or deposited by the same quantity of electricity is proportional to the atomic masses divided by the valencies of their ions."
Faraday's second law of electrolysis can also be stated as under:
"The mass of different substances liberated or deposited by the same quantity of electricity is proportional to the atomic masses divided by the valencies of their ions."
Explanation:
Take three solutions of electrolytes: AgNO3, CuSO4 and Al(NO3)3 in a series, pass some quantity of electricity through them for the same time. Now Ag Cu and Al metals collect at the cathode. Their masses are directly proportional to their equivalent masses.
According to Faraday, if 96,500 Coulombs (or 1 Faraday) is passed through these electrolytes,
we get
Ag, Cu and Al respectively.
.
I found that very interesting and understand-able.
ReplyDeleteEasy to learn for every student probably the best for exams
ReplyDeleteReally nice lucid explanation. Amazing!
ReplyDeleteThis comment has been removed by the author.
ReplyDeleteVery easy to understand
ReplyDeleteIts really helpful
ReplyDelete